
LabTurbo Biotech
We empower high-complexity laboratories with fully automated molecular extraction and qPCR solutions, while supporting physicians and healthcare providers with rapid, dependable PCR diagnostic testing that drives better clinical decisions.
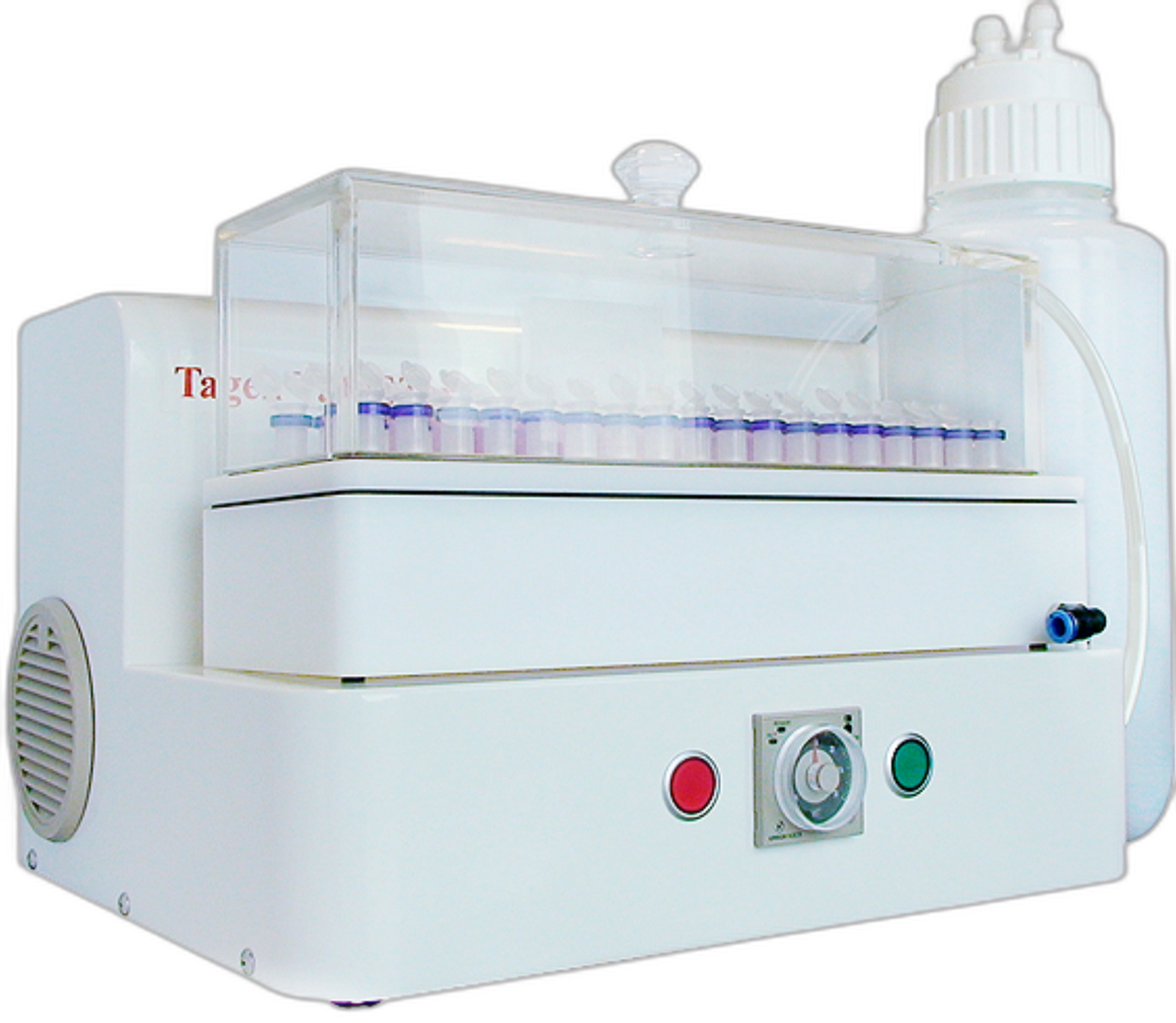
LabTurbo Automated Instruments
Fully automated extraction and qPCR platforms designed for high-throughput, hands-off workflows and consistently reliable results.
Explore Systems


High-Performance PCR & Nucleic Acid Prep Kits
Complete, validated molecular solutions designed for sensitivity, reliability, and seamless integration into your diagnostic workflow.
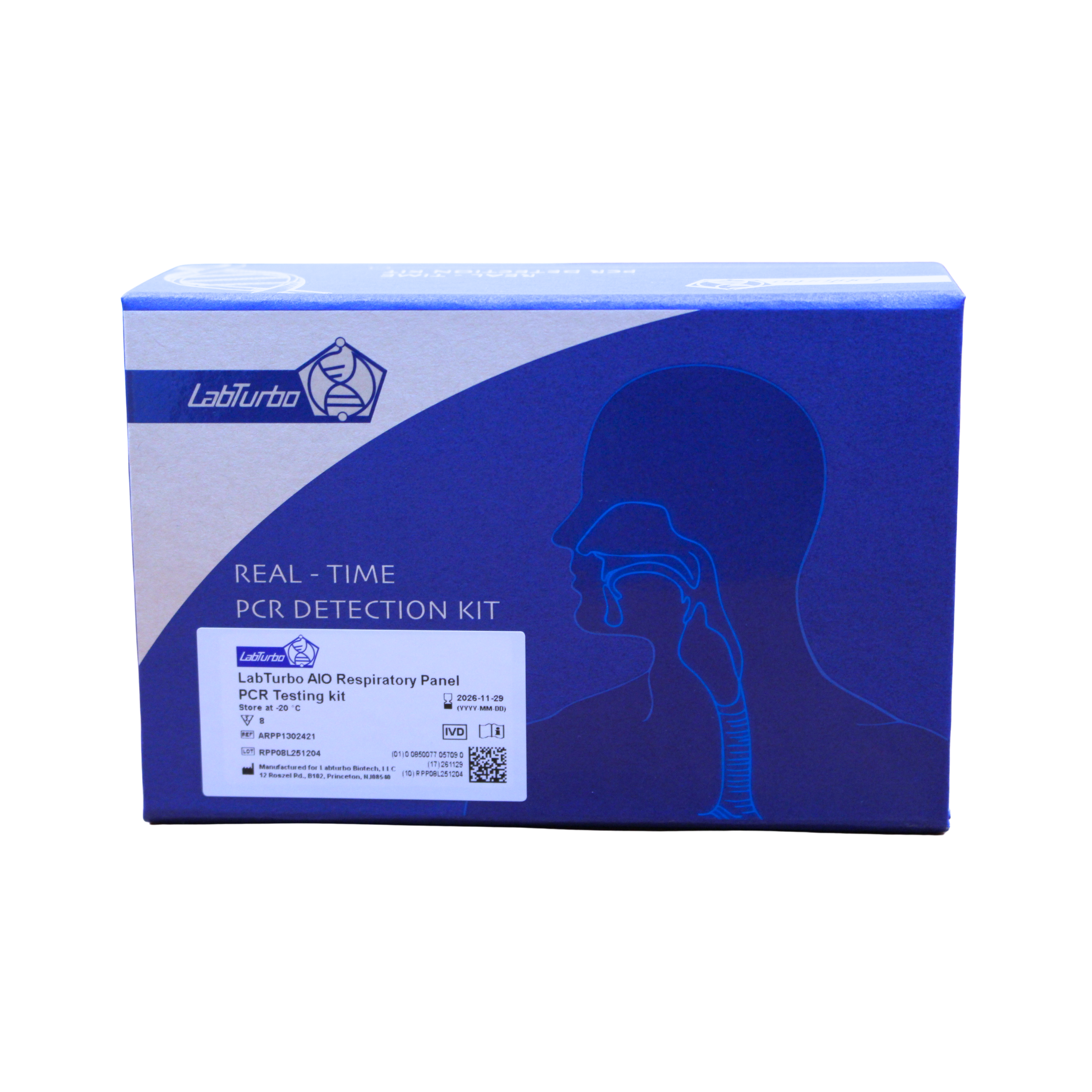
LabTurbo PCR Kits
Validated PCR panels engineered for sensitivity, specificity, and streamlined workflows including RPP, FLU, UTI, STD, and more.

LabTurbo DNA/RNA Extraction Kits
High-yield, high-purity kits custom built for our systems and optimized for a range of sample types — including viral, stool, forensic, and plasmid sources.

Favorgen Manual DNA/RNA Extraction Kits
Reliable manual extraction solutions for flexible, small- to mid-volume workflows.
Comprehensive Clinical Testing Services
Designed for accuracy, speed, and scalability — LabTurbo’s clinical testing panels support high-complexity labs with validated targets for key health conditions.
See all services →COVID, FLU, RSV

UTI
STD
Respiratory Pathogens
GI
PGx
Latest News
Explore the latest from LabTurbo: innovation highlights, launches, and events.
Customer
Testimonials
Read what our clients have to say